What if your antidepressants could work wonders in just three days? Introducing Zuranolone
Gone are the gruelling weeks of waiting for antidepressants to kick in since a new drug meant to help those struggling with depression has just been proved to improve symptoms after a mere matter of days, according to a new report by Reuters.
Trialling several antidepressants can be a nightmare, to put it simply. It generally takes a couple of weeks to actually feel an uptick in your mood after administering them and to get anything that isn’t the same old black cloud over your head can be a miracle sometimes. Understandably, many are put off by drug therapy for this very reason. In my experience, high functioning or not, the adjustment period of drug therapy was worse than the condition I wanted treatment for in the first place.
Drug therapies are often the definition of “it gets worse before it gets better.” But no amount of singing along to ‘Getting Better’ as The Cat in The Hat taught me to do can get you through weeds with antidepressants. But worry not as it looks like the depressing dog days might soon be over with the newest advent of antidepressant Zuranolone, which has been revealed to ease symptoms of depression after just as little as three days.
That’s right, folks. The prolonged period of drug effectiveness could soon be no more. Making waves in the scientific community towards the end of 2021, it was announced on Wednesday 16 February by biopharmaceutical company Sage Therapeutics and global biotechnology companies Bigoen that ‘Phase Three’ of their ongoing study has met its main goal. This part of the study sought to compare 50 milligrams of Zuranolone with standard care antidepressants against placebo and standard care antidepressants in people that have major depressive disorder (MDD), a specific type of depression that can severely affect the day to day life of those who suffer from it.
Zuranolone has already been tested in clinical trials for postpartum depression. Stage three of Sage Therapeutics’ trials included 440 participants who were diagnosed with MDD, which affects 16 million American adults every year, according to Reuters. The main treatment offered, in terms of drug therapy, comes in the form of antidepressants, which can take up to six weeks to show effect. The trial split the participants up in two groups—some were offered Zuranolone with a side of other antidepressants while others were given a placebo as well as antidepressants.
The results revealed that those who received Zuranolone paired with other common antidepressants noticed a positive change in their depressive symptoms. After just a matter of days, an array of symptoms including anxiety and sadness were alleviated. In comparison, the placebo group who only took standard antidepressants did not report this same change in mood.
Though we all may want to run to the nearest pharmacy after hearing this, we’ll have to considerably slow our pace—as Zuranolone is still in its early trials. However, the research is promising with these recent findings accelerating the potential of faster-acting medication to aid those in need and avoid the worst cumulative effects of depression (which can even include suicidal ideation and attempts).
Despite the extreme difference in the short run, the study did not find any significant differences in the effects of Zuranolone in the long-term after 42 days. Such concerns of durability had a hand in the dramatic plummet of Sage Therapeutics’ stock earlier this week. Among all the buzz, analysts have called into question the commerciability of the drug product, as initially reported in Mic.
Nonetheless, research into a faster-acting drug therapy for those with depression is necessary in the field. We can’t possibly avoid the elephant in the room: the COVID-19 pandemic and how rates of anxiety have skyrocketed because of it. Mental health services have long been stretched thin, but the added strain of the pandemic has meant that many are going without the care they desperately need.
There is an influx of people seeking mental health support currently. One study, published in The Lancet medical journal in October 2021 looked at the global prevalence of depression and anxiety disorders in 204 countries in 2020 due to the COVID-19 pandemic. According to CNBC, the study found that mental health took a nosedive “with an estimated 53 million additional cases of major depressive disorders and 76 million additional cases of anxiety disorders seen globally.” Results also showed that women and younger people were more affected than men and older adults.
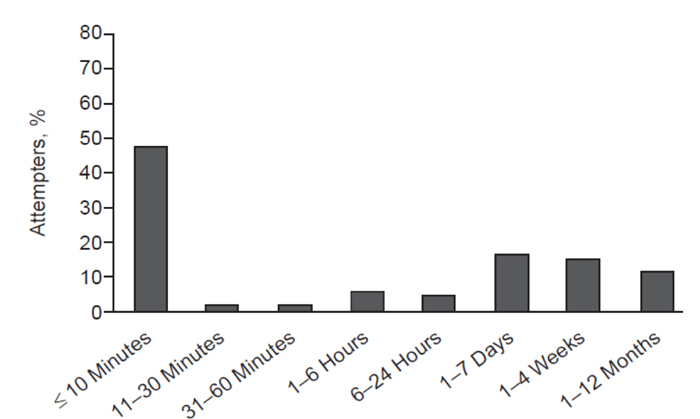
The process of receiving a course of drug therapy in itself is long and taxing as there are multiple barriers one needs to break through in order to receive treatment—be it time, money or practicality. The weeks-long wait to receive antidepressants or for their effectiveness to even begin can be deadly for some. Another study conducted in Houston that observed the duration of suicidal crises by interviewing 153 survivors ranging in age from 13 to 34 found just how slim the line between attempt and completion really is. When asked “How much time passed between the time you decided to complete suicide and when you actually attempted suicide?” the study uncovered a distressing answer. One in four (24 per cent) said they only deliberated for less than five minutes.
A different study of people seen in a hospital following a suicide act revealed that 48 per cent said they had first thought of attempting within 10 minutes before actually conducting the attempt. Figure below shows the rest of the results from the study conducted in Houston.
In lieu of recent events surrounding Zuranolone, Sage Therapeutics is still seeking its next steps with plans for an application for FDA approval by the second half of 2022. Hopefully, the biopharmaceutical company can get the ball rolling before the colder months return and seasonal depression rears its ugly head in our vitamin D-deficient lives. Because, as we know by now, time is of the essence…





